Featured
- Get link
- X
- Other Apps
Electrochemical Cell Potential Can Be Calculated Using The Nernst Equation.
Electrochemical Cell Potential Can Be Calculated Using The Nernst Equation.. So the cell potential e is equal to the standard cell potential e zero minus.0592 volts over n times the log of q where q is the reaction quotient. Since active coefficients in dilute solutions are close to unity, nernst equation can be given solely in terms of concentrations.
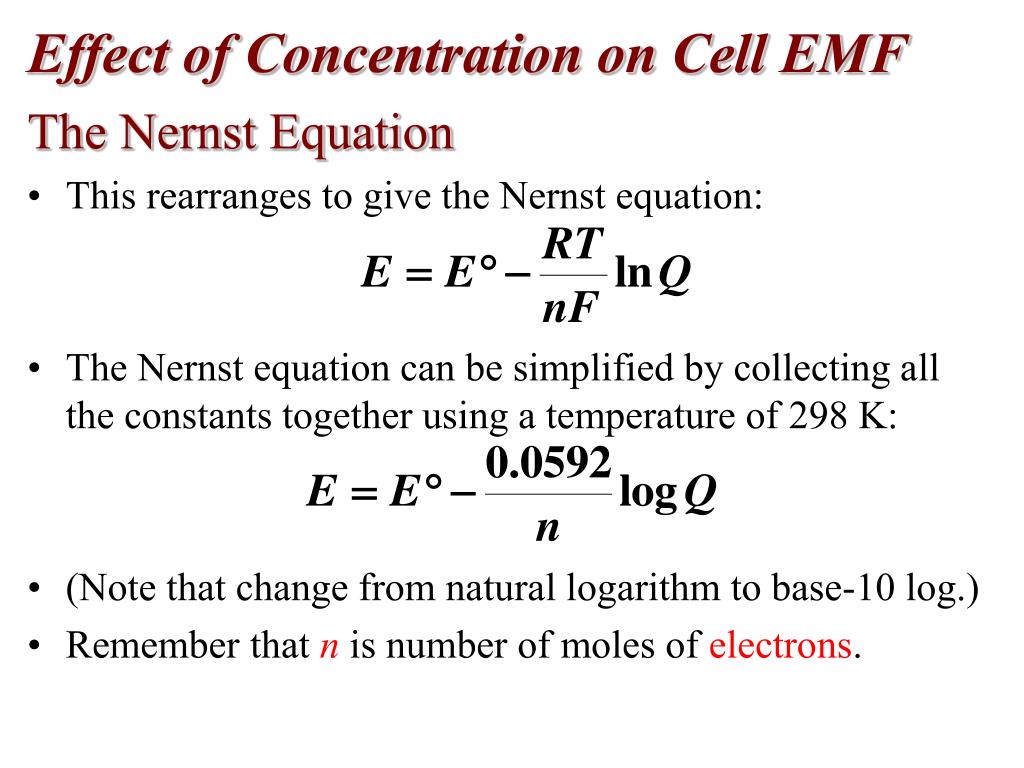
Since active coefficients in dilute solutions are close to unity, nernst equation can be given solely in terms of concentrations. Also, used in ph measurements. What are the limitations of the nernst equation.
(F)Lnq Cell 9 Identify The Value Represented By Each Variable In The Equation.
The resting membrane potential, or baseline state, of a cell, is calculated using this equation. The nernst equation allows the calculation of an electrochemical cell using the standard cell potential, the temperature, the number of electrons transferred in the cell, and the reaction quotient. The cell potential under standard conditions is 0 as the oxidation is +0.76v and the.
Also, Used In Ph Measurements.
7 electrochemical cell potential can be calculated using the nernst equation. Δ g 0 = − n f e c e l l 0 δ g = − n f e c e l l putting these two values in above equation: The nernst equation is used to find the potential of an electrochemical cell or an oxidation/ reduction reaction under conditions of changing temperature and concentration of the electrolyte.
This Question Can Be Solved By Using The Nernst Equation At Standard Conditions:
1.00 electrochemical cell potential can be calculated using the nernst equation. (0.04 m)zn(s)|z… get the answers you need, now! These are devices that convert the chemical energy of some redox reactions to electrical energy.they are also called galvanic cells or voltaic cells.
Using The Values Of Concentrations In The Concentration Cell We Can Work Out Q And From This Using Rt And The Number Of Moles Of Electrons In This Example 2 We Can Calculate The Cell Potential.
Ecell = e ∘ cell − 0.0257 v n lnq or ecell = e ∘ cell − 0.0592 v n logq. Nernst equation is given as: Click here👆to get an answer to your question ️ calculate using nernst equation cell potential of the following electrochemical cell at 298k.
At Temperature T, Change In Gibbs Free Energy Is Given By:
Helpful in calculation of ion’s potential having the charge “z” over the membrane. It is constructed by dipping a zn rod in znso 4 solution and a cu rod in cuso 4 solution. Δ g = δ g 0 + r t l n q where, q = reaction quotient, r = universal gas constant.
Popular Posts
Standard Costs Are Used In The Calculation Of
- Get link
- X
- Other Apps
Comments
Post a Comment